
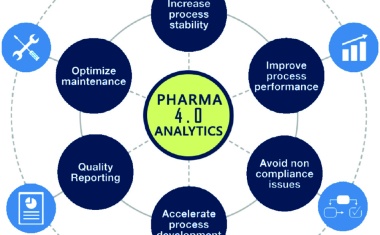
Pharma 4.0—the Key Enabler for Successful Digital Transformation in Pharma
Part 3: Seven Theses for successful Digitalization in Pharma

Part 3: Seven Theses for successful Digitalization in Pharma

Part 2: Digital transformation - 10 Years after the Start of Pharma 4.0

Part 1: Building a Business Case for Pharma 4.0

Industry experts Michelangelo Canzoneri, Josef Trapl, Wolfgang Winter, Christian Woelbeling, and Thomas Zimmer – all members of ISPE’s Pharma 4.0 guide core team – explain the transformational challenges and critical success factors of the digital Pharma 4.0 journey.
Industry experts Josef Trapl, Wolfgang Winter, Christian Woelbeling, and Thomas Zimmer – all members of ISPE’s Pharma 4.0 group – talk about the idea behind ISPE’s Pharma 4.0 initiative and the challenges on the way to realize the digital transformation of the pharmaceutical industry.




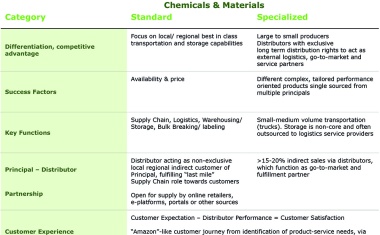
The traditional business model of chemical distributors is currently fundamentally challenged on one side by digitally enabled activities from principals, logistics providers, customers, start-ups, larger chemical distributors or combinations of smaller players, who try their own online channels, and on the other side by general e-retailers like Alibaba or Amazon, who explore to enter also into chemical & material categories.
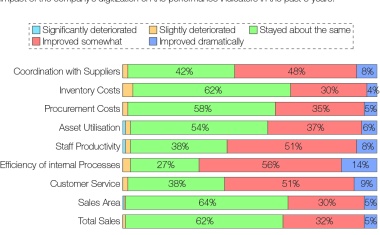
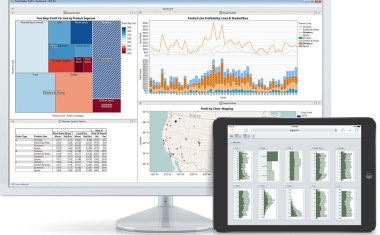



Oil and gas, petrochemical, refining, life sciences and other manufacturing companies face ongoing pressures to achieve improved financial results.


In a joint recent study of 2017 the VDMA Large Industrial Plant Manufacturers' Group and MaexPartners analyzed the potential of Industry 4.0 for large industrial plant manufacturing.















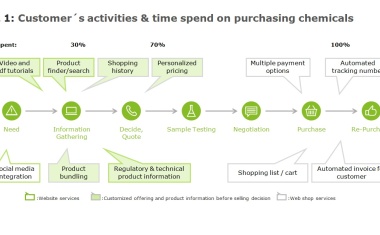
Digitalization in chemicals and chemical distribution is the monetarization of data to significantly cut costs („Operational Excellence“) and to capture additional profitable growth areas (“New Business Models”).







