Small Particles with a Big Impact
In 2022, the European Commission revised the definition of nanomaterials in a new recommendation, which supports a uniform EU regulatory framework and aims at aligning the legislation across various sectors. Therefore, particle product design is an enabling technology relevant for many sectors, such as chemicals, consumer products, foods and beverages, health, energy, and the environment.

Nanomaterials consist of differently shaped tiny particles no larger than one hundred nanometers. As nanomaterials exhibit various promising technological properties, modern particle technology includes promising applications such as printable electronics for the energy sector, or biomedical sensors for the pharmaceutical or medical health industry to name a few. Therefore, particle product design is an enabling technology relevant for many sectors, such as chemicals, consumer products, foods and beverages, health, energy, and the environment.
In the European Union, all nanomaterials are covered by the same rigorous regulatory framework that ensures the safe use of all chemicals and mixtures, i.e., the REACh and CLP regulations. In 2022, the European Commission revised the definition of nanomaterials in a new recommendation, which supports a uniform EU regulatory framework and aims at aligning the legislation across various sectors. The revised definition of the term “nanomaterial” is deemed to be very technical and is defined as follows: A nanomaterial is a natural or manufactured material consisting of solid particles either on their own or as identifiable constituent particles in agglomerates. Moreover, 50% or more of these particles in the number-based size distribution are in the size range below 100 nm including particles with an elongated shape, such as rods, fibers or tubes, or plate-like particles.
In this article, we summarize the impact of the revision of the definition of nanomaterials on companies and their business processes. We highlight which sectors must deal with challenges arising from the new recommendation such as the translation from technical definitions into the business context. One particular challenge includes the reclassification of well-established materials (such as a specific product variant of titanium dioxide, which has been reclassified as non-toxic) as toxic due to its reclassification as a nanomaterial without any change in the product. This is in particular difficult to address as the exact determination of the particle size distribution of a particle ensemble remains challenging and highly depends on the measuring principle. Finally, future possibilities are highlighted together with our partner LUM GmbH, who is active in the development of measurement principles and devices to address the topic of nanomaterial classification directly at the core.
Particle Technology and Nanomaterials Across Various Industrial Sectors
Modern particle technology plays a key role in various sectors such as printable electronics for the energy sector, biomedical sensors for the pharmaceutical or medical health industry and consumer products for foods and beverages. From the industrial perspective, nanomaterials are comprehensive materials produced or manufactured for various fields of application. Among the many different products, such as carbon nanotubes, silica, copper and aluminum oxide, titanium dioxide (see left panel of Fig. 1) is one of the most prominent examples and a widely applied particulate product in the chemical industry, especially in the construction sector. Further examples include dispersion in paints, solid catalysts and surface coatings. The application of titanium dioxide is frequently discussed in the context of nano toxicity.
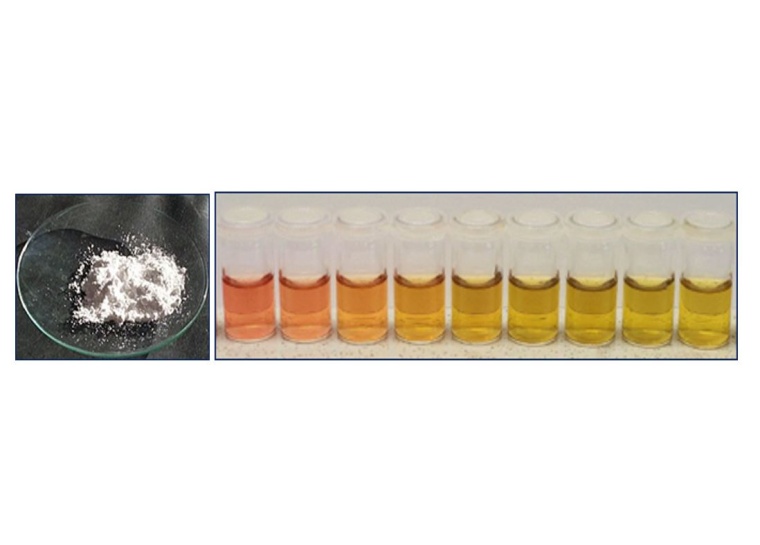
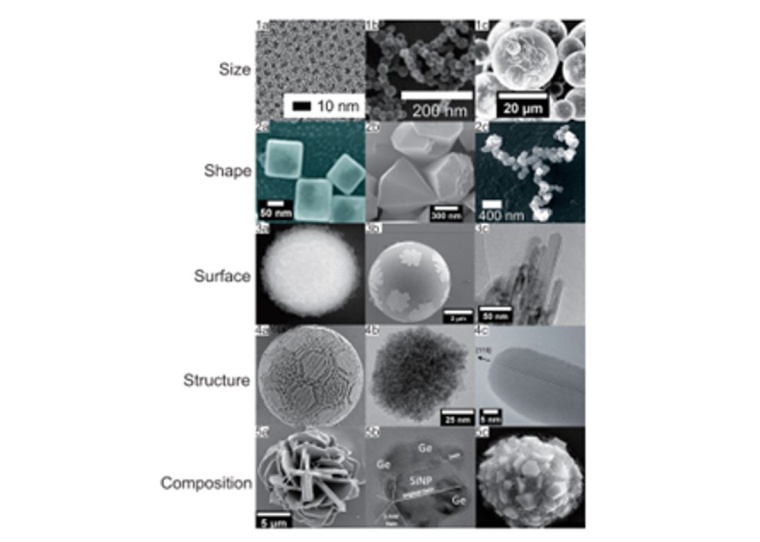
Apart from the industrial point of view, modern particle technology gains a lot of attention from the research and development perspective. While applications such as sensors for medical devices development based on gold-silver nanoalloys (exemplary depicted in the right panel of Fig. 1) are developed from the synthesis point of view, methods for the particle characterization has gained significant importance as the product relevant potential of (nano-)particles directly depend on the physical properties of the respective parties, such as particles’ size, shape and composition, as can exemplary be seen in the right panel of Fig. 1 and was further highlighted in a review article in the scientific literature.
In the context of definition of nanomaterials, certain EU laws require adequate data collection, a thorough risk assessment, as well as labelling of particulate products. This aims to inform customers and consumers of the presence of nanomaterials in products.
In summary, even though modern particle technology triggers innovation, some challenges remain which are discussed and initiated in the context of regulatory frameworks within the EU.
In this article, we highlight the implications of changes of the EU regulations and to detail the direct relationship between meeting the EU regulations of nanomaterials and developing comprehensive particle characterization techniques. Therefore, the next paragraph summarizes the status of the regulations on nanomaterials across the European Union. Finally, we comment on the technical challenges of particle characterization and future possibilities in this area together with our partner LUM.
Regulations on Nanomaterials Within the European Union
The REACh and CLP regulation ensure the safe use of chemicals and their mixtures while enabling competitiveness within the chemical industry. Under REACh, the burden of proof is placed on each company, hence the safe application of each chemical must be demonstrated to ECHA by producing companies in the European market. Conclusively, changes within the regulatory framework of nanomaterials have a direct impact on various companies throughout the entire chemical industry across the entire European Union. As clear guidelines from the regulatory perspective pave the way for advancements and technical developments, an aligned legislation across various sectors will be key for the way forward. Most EU legislations (e.g. REACh, Biocidal Products Regulation, Medical Devices Regulation) and some national legislation use the common definition from Commission Recommendation 2011/696/EU, while the food and cosmetics sectors still relates to an individual definitions of nanomaterials. Since 2020, legal requirements under REACh are applied for manufacturing or importing companies of nanomaterials and particulate products which are considered nanoforms. These requirements address specific reporting obligations which are related to the REACh regulation. In 2022, the definition of nanomaterials was revised with a new recommendation by the European Commission, which aims at supporting a uniform regulatory framework throughout the European union as a deliverable of the Chemicals Strategy for Sustainability. The update refers to the previous recommendation 2011/696/EU taking into account the progress from the scientific community. Notably, 2011/696/EU clearly states that a nanomaterial is considered “a natural, incidental, or manufactured material containing particles.” However, in order to evaluate whether or not a solid powder, i.e. particles or dispersions must be considered a nanomaterials in order to meet the compliance criteria, the technical definition as well as the impact from different particle characterization strategies must be taken into account.
Technical Point of View and Relationship to EU Regulation
From a chemical engineering point of view, in terms of definition, a nanomaterial is a natural or manufactured particulate product or material, which consists of solid particles or dispersed particles in a liquid either on their own or as identifiable constituent particles in agglomerates. Moreover, nanoparticles are usually associated with a broad spectrum of size, shape and composition, which is illustrated in Fig. 2.
Nanomaterials are by definition particles no larger than 100 nm in one dimension in case the nanomaterial is not spherical. However, nanoparticle ensembles are usually characterized by particle property distributions since it is not possible to quantify the properties by one number only. Therefore, the definition of nanomaterials is further refined according to the following description: A material is considered a nanomaterial in case 50% or more of these particles in the number-based particle size distribution are in the size range below 100 nm including particles with an elongated shape, such as a rods, fibers or tubes, or plate-like particles.
Conclusively, comprehensive particle characterization techniques are key to ensure all nanomaterials are in compliance with European and national regulations. As an example, the increase of toxicity is often associated with a decrease of the particle size for materials such as titanium dioxide. The direct determination of the particle size is a tedious task and often requires time consuming sample preparation and the subsequent use of multiple measurement devices with clear standard operation procedures. Moreover, the direct measurement result must be easily understandable with clear guidelines and criteria for lab reports. One particular example in this area is the reclassification of a well-established product, i.e., a specific product variant of titanium dioxide. Titanium dioxide was considered toxic after classification as a nanomaterial without any change in the product but in the regulation. After rigorous discussions on this topic and the support of comprehensive particle characterization techniques, which was in this case provided by LUM/Dr. Lerche, titanium dioxide has been reclassified as non-toxic. A further example is the case of tricalcium citrate (see right panel of Fig. 3), which is a food additive with a large-scale production. As tricalcium citrate shows a plate like structure on the particle level (see left panel of Fig. 3), it needs to be evaluated whether or not the plate thickness is the key property which must meet the compliance criteria or if an equivalent hydrodynamic sphere must be calculated and needs to comply with the EU regulation. Needless to stay, the product, i.e., tricalcium citrate, as well as the way of production did not change and is a key product in the food and consumer industry associated with a large turnover for certain companies.

From our point of view, it becomes clear with this example, that highly accurate and high-throughput particle characterization techniques are one key aspect in the field of nanomaterials. Furthermore, the range of nanomaterials reaches across various industry sectors. Therefore, our partner LUM contributes to future solutions by developing particle characterization techniques further with clearly defined standards and standard operating procedures. With this, the topic of nanomaterial classification is addressed directly at the core.
References to this article can be requested from the authors.
Jens Raschke, Partner, and Maximilian Uttinger, Consultant, BearingPoint, Germany
Dietmar Lerche, CEO, LUM GmbH, Berlin, Germany






















