Astellas Acquires Xyphos Biosciences
Japanese drugmaker Astellas Pharma has boosted its immuno-oncology capabilities with the purchase of US-based Xyphos Biosciences. The acquisition was the second last month for the Tokyo-based pharma, which had also agreed to buy US gene therapy company Audentes Therapeutics for $3 billion.
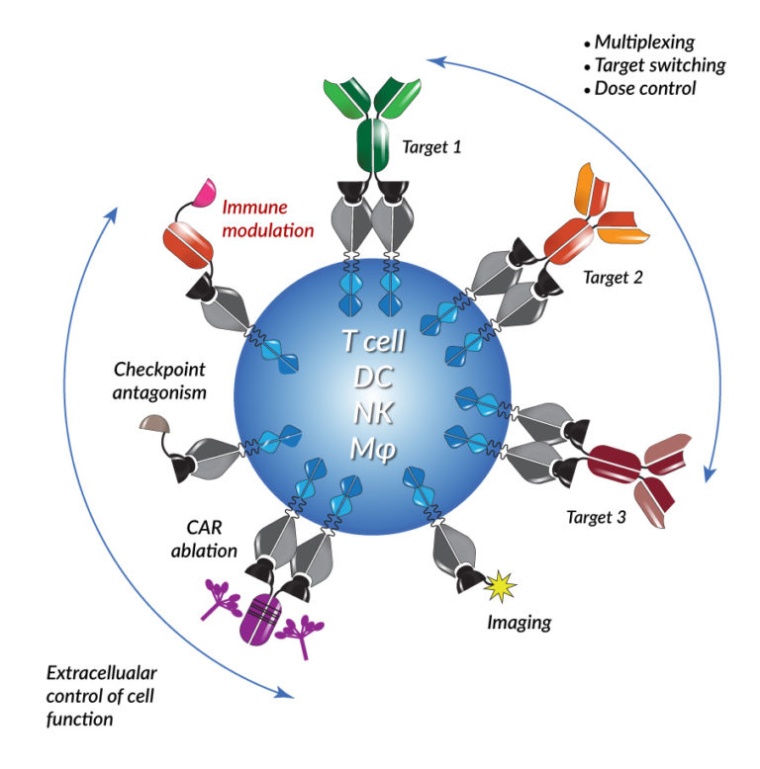
The deal, which is worth up to $665 million, gives Astellas access to Xyphos’s novel ACCEL (Advanced Celllular Control through Engineered Ligands) technology platform.
“Combining this technology with our capabilities in cell therapy that we have been working on so far, we can create next-generation high-function cells and maximize the value of our technology,” said Astellas president and CEO Kenji Yasukawa.
Xyphos’s proprietary molecules can be delivered to natural immune cells or to engineered Chimeric Antigen Receptor (CAR) cells to generate immunotherapies for oncology. Xyphos’ first CAR-T cell product candidate is in preclinical development and scheduled to be tested in a first-in-human clinical study in 2021.
Astellas paid $120 million upfront and will pay the rest on future development milestones being met.