Minimum Requirements in Cell-Based Assays

Cell-based assays have become the preferred alternative to biochemical assays for screening new drug targets. This increase in demand is due to the accurate representation that cell-based assays offer and the possibility of real-time monitoring of cell behavior. The species of origin and cell types used in cell-based assays are often influenced by the drug target that is being investigated. However, regardless of the model system chosen, processing of cultures at each step of the manufacturing process should be standardized and validated for consistency. Assay responsiveness to test compounds can be influenced by many factors including culture medium, doubling time of cells, passage numbers, microbial contamination, and misidentification of cells. These inconsistencies in cell authenticity and quality have led to false results and render invalid comparisons of data from disparate laboratories with shared research interests.
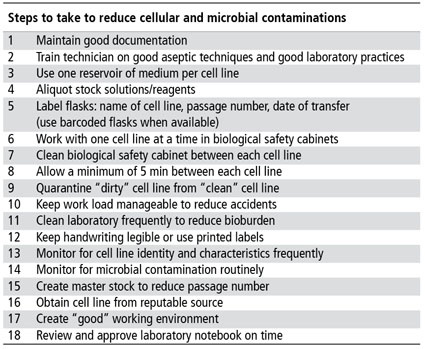
This article takes a comprehensive overview in highlighting how unreliable results can be traced back to poor tissue culture practices such as (1) unverified morphology, (2) suboptimal growth conditions, (3) variable performance of cells at high passage numbers or population doubling levels (PDLs), (4) microbial contamination, especially mycoplasma, (5) incorrect species, cellular cross-contamination and misidentification of cell lines and (6) loss of functional performance of the cells.
Morphology
Observing cell morphology is frequently ignored, yet microscopic observation of cells is the most powerful initial step in becoming acquainted with cells in culture. It is important that the cell culturist monitors regularly the health of the cell in culture, including the morphological changes of the entire cell (shape, size), as well as the properties of multi-cellular colonies. As cells age, they undergo progressive morphological changes, in vitro - the senescing cells become larger and the senescent population acquires a more diverse morphology [1].
Sterility Testing
(Microbial Contamination)
Microbial contamination (bacteria, fungi, mycoplasma and viruses) of cell culture systems remains a major concern. In most instances, microbial contamination is overt and is visible with the naked eye. However, low-levels of contamination of the more fastidious bacteria and fungi are often missed by many laboratories.
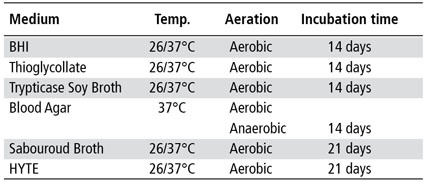
The inclusion of a series of microbial culture tests allows detection of the most common species of bacteria and fungi that persist in cell culture systems. For example, one can use a regimen of bacteriological culture media to reveal a wide variety of microorganisms that can survive as contaminants in cell lines or growth media [2] (table 1). Detection of mycoplasma infection is not noticeable by routine microscopy. Many mycoplasma grow very slowly in continuous cell culture and often go unrecognized since they do not change the cells or the medium. Mycoplasma infection has been known to interfere with studies of metabolism, receptors, virus-host interactions, cell divisions, and others, virtually invalidating research findings. The most commonly performed methods for the detection of mycoplasma are the Hoechst stain (indirect), the agar method (direct) and PCR [3-6].
Detection of endogenous and contaminating viruses in a cell culture system is the most problematic type of microbial contamination. The presence of a characteristic cytopathic effect (CPE) provides an early sign of viral contamination. However, the presence of latent infectious viruses in cell culture remains undetected until the appropriate test methods are applied. Most common methods for detecting viral contamination are immunostaining, ELISA and PCR.
Optimal Growth Conditions (Growth Curve)
Establishing a growth curve is one of the most useful tools for determining the optimal growth condition of a cell line. Counting cells at intervals after subculture and plotting the total number of cells versus time to establish a growth curve can provide several key characteristics such as lag phase, exponential phase and saturation phase [7, 8]. Given that the growth conditions are constant, these phases are characteristic of each cell line and when applied will give consistent and reproducible results [9].
Effect of High Passage Numbers on Cell Line Performance
When placed in culture, most diploid cells will divide rapidly and are often subcultured several times to reach an average of fifty cumulative population doublings (CPDs) before they die or senesce (replicative senescence, RS). This phenomenon is known as "Hayflick's limit". Most cell lines derived from tumor tissue will overcome the "Hayflick's limit" [10, 11]. However, extended propagation and subcultivation of diploid cell lines and tumor cell lines induce a selective pressure that may result in genotypic and phenotypic instability [12, 13]. Long term passaging of cells can impact cell function [14-16] and so it is important to establish the minimum acceptable passage to ensure reproducible results.
Species Identification
(Cellular Cross-Contamination and Misidentification of Cell Lines)
Historically, the most commonly used tests to determine the species of origin of animal cell lines, include HLA typing, immunophenotyping, isoenzymology [17] and karyotyping. More recently, cytochrome oxidase subunit I (COI) [18, 19] has been applied for interspecies identification and STR analysis for intraspecies identification of human cell lines.
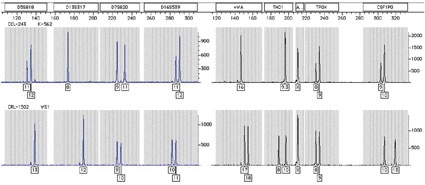
Interspecies identification of animal cell lines by COI analysis relies on a multiplex PCR assay that rapidly identifies 14 of the most common animal species in cell culture (fig. 1). The protocol involves amplification with a universal primer set that targets the 640 bp COI consensus sequence. Each species is characterized by a specific amplicon size, which is used for identification. The procedure can be extended to identify unknown species by sequencing the amplicons and comparing these to sequences of voucher specimens [19].
STR (DNA profiling) analysis is the most commonly used method for human cell line identification [20-23]. This method involves the simultaneous amplification of multiple STR markers (i.e. VWA, CSF1PO, TPOX, D13S17, D5S818, D18S51, D16S539) and the amelogenin gene for gender determination. Using a multiplex PCR reaction of eight markers, the STR markers can discriminate one human sample from another at a level of 1 x 10-9. Each unique human line has a distinct DNA profile (fig. 2) and the method can be used to detect contaminating human cells at a level as low as 10 % [23]. The procedures described above are not only useful for species identification, but are also employed for detecting cross-contaminating or misidentified cell lines [22, 23].
Functional Performance of Cell Line
It is highly recommended that during the propagation of a cell line and, indeed, before publication, functionality and/or a unique characteristic test is performed to confirm previous results. It is critical that these unique cell traits are maintained during propagation and while experiments are being performed.
Summary
One of the essential dogmas of cell culture is that diploid and continuous cell lines will change during long term propagation. Whether these changes are due to increased passages, suboptimal growth conditions, cellular-cross contamination, loss of functionality or microbial contamination (table 2), it is important that a comprehensive approach be taken to reduce these changes. Ignoring any one of these factors will lead to inconsistent or irreproducible results, loss of cell lines, loss of time and money, misrepresentation of scientific
information in the public
domain, discordant or irreproducible results, private embarrassment and public humiliation.
References are available from the author.
most read

Q1 2025 Chemical Industry: Diverging Trends
The first quarter of 2025 highlights a continued divergence between the European and US chemical industries.

20 Years of CHEManager International
Incredible but true: CHEManager International is celebrating its 20th anniversary!

Pharma 4.0—the Key Enabler for Successful Digital Transformation in Pharma
Part 3: Seven Theses for successful Digitalization in Pharma

Pharma 4.0 – the Key Enabler for Successful Digital Transformation in Pharma
Part 1: Building a Business Case for Pharma 4.0
US Tariffs Fatal for European Pharma
Trump's tariff policy is a considerable burden and a break with previous practice.






