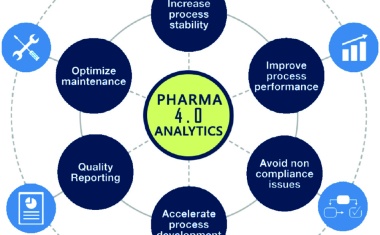
Pharma 4.0—the Key Enabler for Successful Digital Transformation in Pharma
Part 3: Seven Theses for successful Digitalization in Pharma

Part 3: Seven Theses for successful Digitalization in Pharma

Part 2: Digital transformation - 10 Years after the Start of Pharma 4.0

Part 1: Building a Business Case for Pharma 4.0

Industry experts Michelangelo Canzoneri, Josef Trapl, Wolfgang Winter, Christian Woelbeling, and Thomas Zimmer – all members of ISPE’s Pharma 4.0 guide core team – explain the transformational challenges and critical success factors of the digital Pharma 4.0 journey.

Artificial intelligence (AI) has become one of the supporting pillars for digitalization in many areas of the business world. The pharmaceutical industry and its GxP-regulated areas also want to use AI in a beneficial way, but only a small fraction of companies follows a systematic approach for the digitalization of their operations and validation. However, the assurance of integrity and quality of outputs via computerized system validation is essential for applications in GxP environments.
ISPE and its members are developing the roadmap to introduce Industry 4.0, also called the Smart Factory, at the pharmaceutical industry as Pharma 4.0 – an operating model that is interconnected, meaning that the digital tools allow for a fully connected network to enable direct communication between all levels in an organization.
Industry experts Josef Trapl, Wolfgang Winter, Christian Woelbeling, and Thomas Zimmer – all members of ISPE’s Pharma 4.0 group – talk about the idea behind ISPE’s Pharma 4.0 initiative and the challenges on the way to realize the digital transformation of the pharmaceutical industry.