US Government Buys 3 Million Novavax Doses
The US Department of Health and Human Services (HHS) and the Department of Defense (DOD) have ordered 3.2 million doses of Novavax’s Covid-19 vaccine, even before the protein-based and adjuvanted shot has cleared the US Food and Drug Administration.
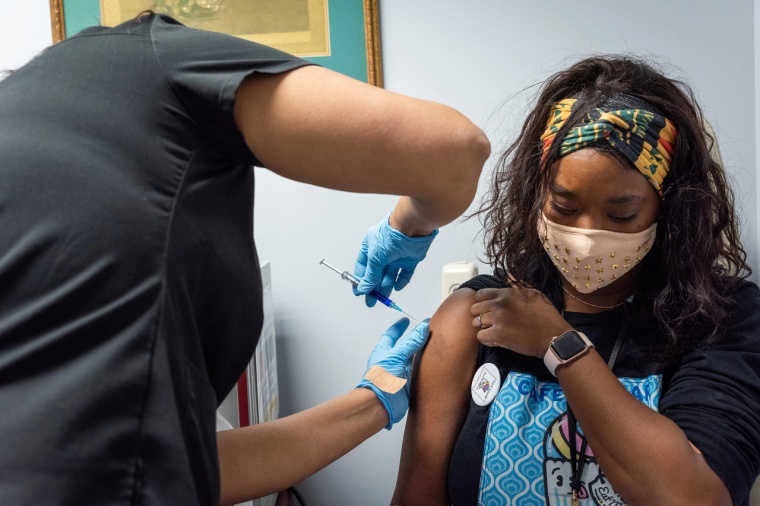
In June, the FDA’s advisory panel of independent health experts recommended that the candidate branded as Nuaxovid be approved but while the health agency generally follows the panel’s advice, up to now it has declined to comment on when it might act.
Some reports said the FDA was scrutinizing the vaccine carefully in consultation with Novavax as it would be the first of its type authorized for sale in the US. The Pfizer/BioNTech and Moderna vaccines are mRNA-based and the one made by Johnson and Johnson’s pharma subsidiary Janssen is an adenovirus vector vaccine.
Novavax has said it anticipates completing all necessary quality testing in the next few weeks, which would support final release of the product. If the vaccine is approved, the two government departments will provide it at no cost to states, jurisdictions, federal pharmacy partners and federally qualified health centers.
US press reports quote Jason Roos, COO of HHS Coordination Operations and Response Element (H-CORE), as saying the department will work to ensure that anyone eligible who wants a vaccine can get one.
“While more than two-thirds of the American public are already fully vaccinated, we must maintain a sense of urgency to ensure all eligible individuals get vaccinated, particularly heading into the fall,” Roos said. “This latest vaccine,” he added, would offer people another choice to help protect themselves from severe disease or hospitalization caused by Covid-19.”
Some vaccination campaign organizers say they believe the protein-based shot should be more acceptable to vaccine skeptics than the mRNA products, though opinion polls do not bear this out. In countries where Nuaxovid is already on the market, vaccination numbers have not risen since its rollout.
Monkeypox vaccine distributed to US states
Separately, HHS is making available an additional 144,000 doses of Bavarian Nordic’s Jynneos monkeypox vaccine to states and jurisdictions. The doses of the vaccine approved by the FDA for both smallpox and monkeypox in 2019 are being shipped from the Strategic National Stockpile.
Steve Adams, director of the Stockpile, said 200,000 Jynneos vaccine doses have already been distributed in communities where transmission has been the highest. According to the US Centers for Disease Control CDC), around 700 monkeypox cases have been confirmed in the US to date.
Author: Dede Williams, Freelance Journalist

















