Pharma in the Outcome Era
Blockbusters Not Expected to Set the Tone of the Future
Change Is Happening - Pharma is entering the outcome era. Today, launching a new drug means the companies are forced to make much more effort to prove the drug's additional value and benefits. The obligation to prove the best possible health outcomes and additional benefit is the prerequisite to achieve adequate price and reimbursement. The report "Launch Excellence in the Outcome Era" published recently by Camelot Management Consultants shows that the industry seems not yet to fully realize the advent of this new era with all its consequences.
According to the Camelot study, as many as 30% of respondents still anticipate new product launches in blockbuster style - focused solely on regulatory approval pre-launch and a strong sales force post-launch, with little change to the launch process. However, the report is clear: In order to achieve launch excellence, the entire process of launching must adapt. Changes in timing of the launch phases are already palpable, and even though respondents seem unaware of the extent to which the launch process will be rattled, they already realize some fundamental changes: Every launch phase is strongly affected by an increase of cross-functionality. This increasing cross-functionality is mirrored in the time required for each launch phase. As pre-launch market and stakeholder preparation has become more critical, pharmaceutical companies are initiating their product launch campaigns much earlier than in the past. This fundamental change also extends post-launch: Now companies need to demonstrate long-term, superior health outcome under real-world conditions (fig. 1).
The report further highlights that given the increasing importance of cross-functional collaboration, only clear governance and excellent project management can lead to launch excellence. To build a compelling value proposition, the whole launch team needs to focus early on proving a compound´s additional benefit, ensuring the proper design of clinical trials with valuable endpoints. The market access function builds the insights on where value lies for patients, health technology assessments (HTAs) and payers; also important are the necessary comparators, biomarkers and endpoints. They need to pass on this information to clinical development throughout the entire development process. To achieve the optimum results in later phases, teams have to collaborate to answer questions such as the commercial potential of alternative sub-indications, different market conditions and acceptance for a new drug and consumers' needs. The survey indicates that cross-functional collaboration represents a fundamental change in the way launch teams used to work.
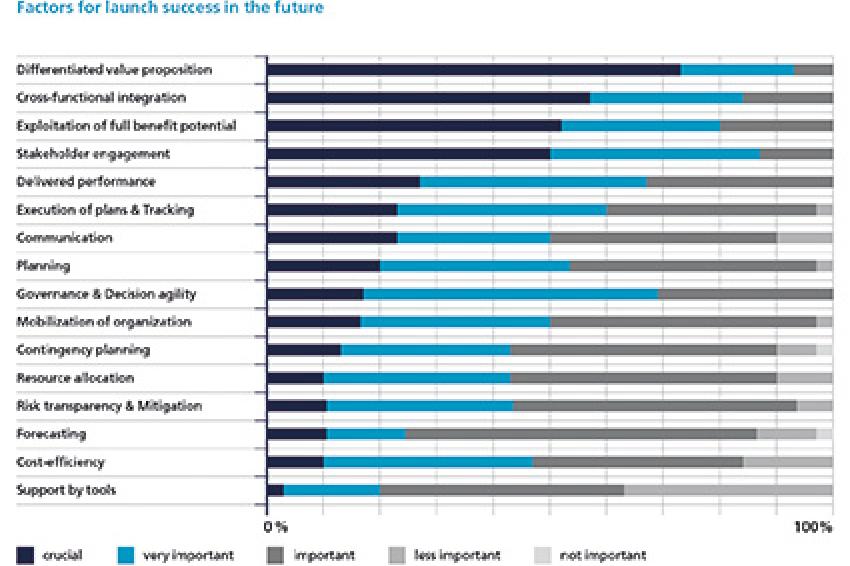
What´s more, results also show that many organizations find it difficult to adapt to such changes. Also the clear definition of roles and responsibilities within an organization ranges among the top launch challenges. Especially against this background, solid governance mechanisms and excellent project management are paramount to ensure well-executed coordination and communication - during all launch phases and across all involved functions. Yet, in terms of governance and decision agility, the survey finds 0% of respondents evaluating launches they keep track of as excellent (fig. 2).
Stakeholder Management as a Launch Strategy
Launch excellence does not only imply outstanding cross-functional collaboration but also a rising consideration of stakeholder management as core element of the launch strategy. Launching in the outcome era is aggravated by an increasingly expanded stakeholder landscape. In total, 96.8% of survey respondents believe this to be a driver of the soaring complexity of the launch process. Beyond the traditional doctors and key opinion leaders (KOLs), the tremendous expansion of the stakeholder landscape ranges from governments, payers and HTAs to patient advocate groups.
Above that, individual stakeholder impact on launch success is on the rise. Payers and patients are increasingly influencing decision-making - they have a say in which drug offers the best value for money in terms of improved health outcomes or economic benefit. Thus, to succeed in the outcome era, excellent market preparation is the key. Stakeholders have to be convinced of the value of the new drug. Ideally, they already await the product at launch. Consequently, respondents confirm an effective stakeholder management is becoming one of the most critical elements in determining future success. Interestingly, at the same time they diagnose "satisfying different stakeholder needs" as the biggest challenge (fig. 3 and 4).
'Do More'
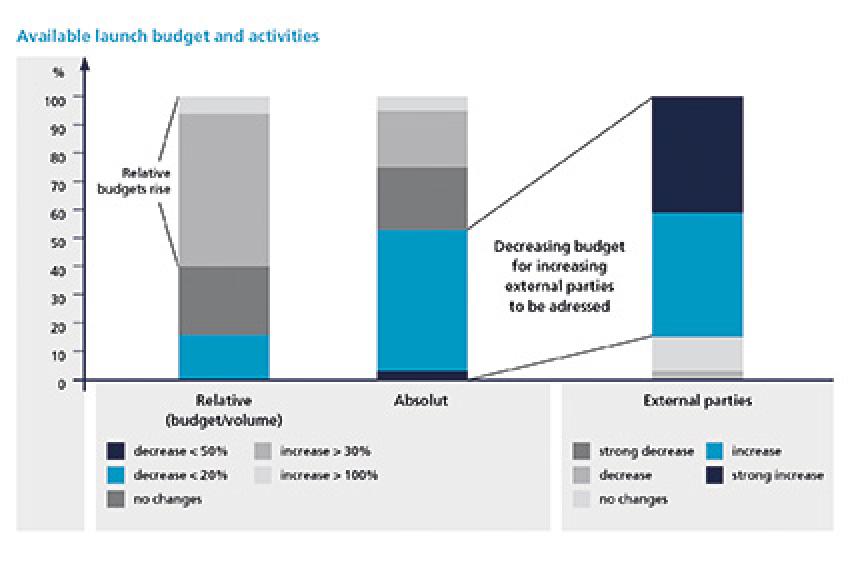
The report shows that launching a drug today requires pharma companies to do more. They need to invest in the compilation of cost-benefit dossiers and construct value arguments to satisfy pricing and reimbursement requirements in different markets. They need to focus a great deal on an elaborate stakeholder management and to ensure excellent market preparation for increasingly parallel market entries. Hence, the survey highlights a growing number of activities and the involvement of many more external parties.
In connection with decreasing revenues and profit margins and the continuous downward price pressure from payers, "doing more with less" should become a growing concern for companies. Correspondingly, the survey found that launch budgets decrease in absolute terms, but rise in relation to the value/volume of launches. Interestingly enough, limited resources seem not yet to be an issue for launch teams and there are still almost a fifth of respondents who claim cost-efficiency as only a subordinate success factor. Also, when asked about their status of excellence in cost-efficiency, respondents' assessment turned out particularly weak - 40% of respondents indicated "fair" and as much as 10% see "room for improvement." However, sooner or later, tight budgets will make it imperative to strive for rigorous cost-efficiency in every step of the launch process. Accordingly, results suggest that 80% of respondents agree an essential aspect to counter constrained budgets is to increase launch excellence (fig. 5).
High Stakes around the World
The stakes in this game are high, with Germany already having implemented its Act on the Reform of the Market for Medicinal Products (AMNOG) price reforms and the UK looking to follow suit in 2014 with its value-based pricing reforms. AMNOG draws a direct connection between added value and the level of reimbursement - through international reference pricing, failure to satisfy the requirement of added benefit will have a domino effect for the price of the new compound in other countries. The report also explains the effects of the outcome era in emerging markets. Countries in the BRIC region are adapting both to U.S. Food and Drug Administration and European Medicines Agency standards and to recent pricing and market access developments. The Brazilian government manages its healthcare spending by comparing market prices to reference prices in other countries and has recently launched the National Commission for Incorporation of Technologies in the Unified Healthcare System (CONITEC). CONITEC is a HTA body which that bases its strict reimbursement requirements on a product's safety, efficacy, cost-effectiveness and impact on the national drugs budget against comparators.
Within this new launch environment successful launches will need to:
- Expand the market, rather than just gain market share
- Re-define therapy guidelines
- Develop excellent cross-functional collaboration and teamwork
- Develop smarter pre-launch activities, with increased investment in the early launch phases
- Have stakeholders awaiting the product with its differentiated positioning at launch
- Win recognition for creating multi-stakeholder value
- Exceed peak sales expectations.
Lastly, respondents did not expect an increase in the number of launches. The industry should be increasing the number of truly innovative launches and introducing them faster if the sector was not to shrink. Companies must be highly alert to the fact that their environment will be undergoing continuous change, which will inevitably force the launch process to adapt.
__________________________________
- Order a free copy of the study: http://www.camelot-mc.com/surveys
- Register to participate in the bi-annual PHARMA Management Radar: www.pharmamanagementradar.com
- Subscribe to Camelot's free newsletter: http://www.camelot-mc.com/en-t/contact/newsletter-registration
Contact
Camelot Management Consultants AG
Theodor-Heuss-Anlage 12
68165 Mannheim
+49 621 86298 0
+49 621 86298 250