Higher Mass Transfer Performance
Improvement of Wettability of Plastic Packings During the Operational Time
Technology - The design of air/water counterflow mass transfer devices with high specific surface polypropylene packings, used to enlarge the transfer surface, is frequently based on the pure-component data of the involved components of water, air and polypropylene. The low surface energies of non-polar thermoplastic material, in particular, indicate bad wetting properties. A vast number of fundamental calculation methods use the critical interfacial tension as an unchangeable material property to determine the wetted surface. As shown below, the values determined under laboratory conditions are distinctly different in practice.
One of the most important requirements of random or structured mass transfer beds is to provide an as large as possible effective surface for the mass transfer. Among other aspects, the effective surface depends on the interfacial tension between the fluid and the material properties of the surface of the transfer elements. The influence of the interfacial tension on the effective surface has been thoroughly analyzed, and corresponding empirical equations have been set up. It is known that water, in particular, because of its high surface tension wets plastic surfaces very badly. In some fundamental works this fact is sufficiently taken into account by material properties. For film type mass transfer elements in plastic it leads to a calculated degree of wettability of less than 50 % of the possible geometrical surface at usual flow rates.
One factor, which is rarely taken into consideration in tests, is the behavior of the interfacial tension over time. After a certain period of time, the wetting properties distinctly improve, thus also improving the degree of wettability. The wetted surface is almost doubled even in the case of clean water, such as drinking water.
Due to its chemical properties, the basic materials of film type packings have certain surface energies. In combination with the surface tension of the fluid applied, a contact angle is produced which is decisive for the wettability of the system.
The surface energy increases with increasing polarity of the surface. In the case of polypropylene, this surface energy is relatively low (30 mN/m (Lake, 2009)). This leads to the fact that media, such as water, which has a high surface energy, does not wet these materials well in the beginning, but forms trickles or drops.
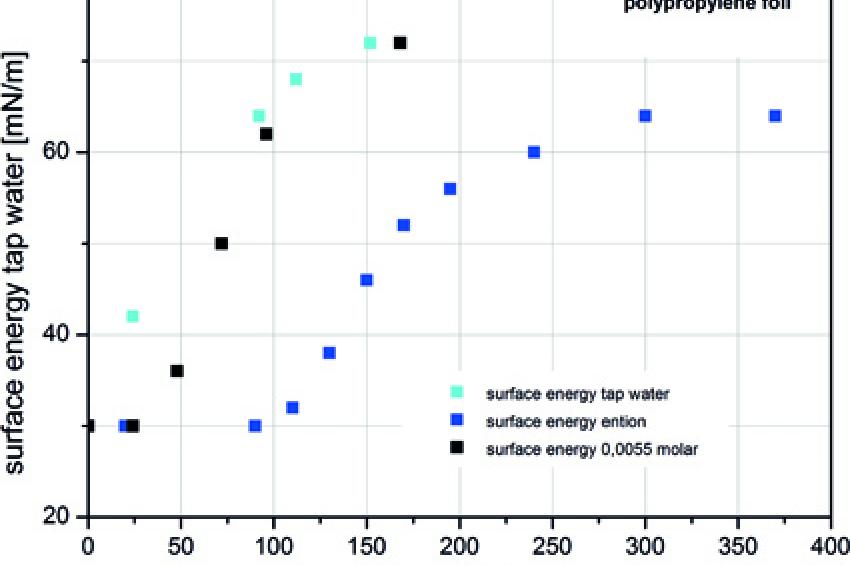
There are, however, processes which cause a change of the surface energy. Apart from technically complex processes, such as a plasma treatment or fluorination, a longer contact with ion-containing water is sufficient to sustainably improve the wettability of polypropylene. In order to demonstrate this effect, foils were laid into an open container with water having different ion concentrations. The test media were:
- tap water with an electrical conductivity of 416 µS/cm
- de-ionized water with an addition of 0.32 g/l of common salt (0.0055 molar solution)
- de-ionized water (in accordance with German standard VDE 0510)
In the process the foils were completely in contact with the water; there was no additional agitation of the water. The foil was taken out of the water at regular intervals and dried upright in air. Subsequently a test ink set (following German standard DIN 53364), which is graduated between 30 and 72 mN/m in steps of 2 m/Nm, is used to determine the surface energy of the foil.
Fig. 1 reveals the results of these tests. In the case of tap water, a very high surface energy is already achieved after 150 hours. In the test with a sodium chloride solution, this process takes only a little longer. In the case of distilled water, the process is slowed down considerably, but at the same time an increase of the surface energy can be measured over time. This shows that the surface energy of a material, which is in continuous contact with aqueous solutions increases in any case, even the open contact of the de-ion with the atmosphere renders this effect.
The wettability of a structured packing has a significant influence on the effective surface and performance, in particular at low irrigation densities. In the case of lower surface energy, distinctly less surface can be used due to the formation of trickles than in case of a higher surface energy so that the performance measurements on new fills (30 m/N/m) and on "established" fills (72 mN//m) may differ considerably.
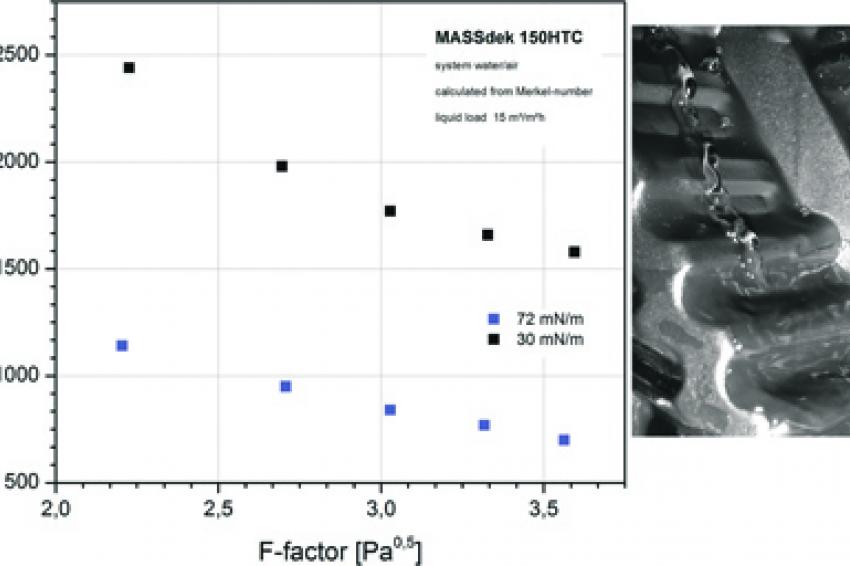
As an example, GEA 2H Water Technologies has carried out heat exchange measurements in countercurrent flow (water/air system) on packing type Massdek 150 HTC, in which the results were converted into HTU values by dividing the height by Merkel's coefficient.
Shown here are the two extremes of surface energy for identical liquid loads of 15 m/h. The HTU value of a new packing with low surface energy in this case is about twice as high as the HTU value of a very well wetted and used packing, as can be seen. Similar results in plastic packing can also be found in the literature (Reuter & Kröger, 2005).
On the right-hand side of Fig. 2, there is an optical clarification of the phenomenon: The upper half of the foil has the initial lower surface energy, whereas the lower half has been correspondingly pretreated and reveals a distinctly higher surface energy. As can be seen, the trickle turns into a thin film immediately at the transition, when the used area of the foil is reached.
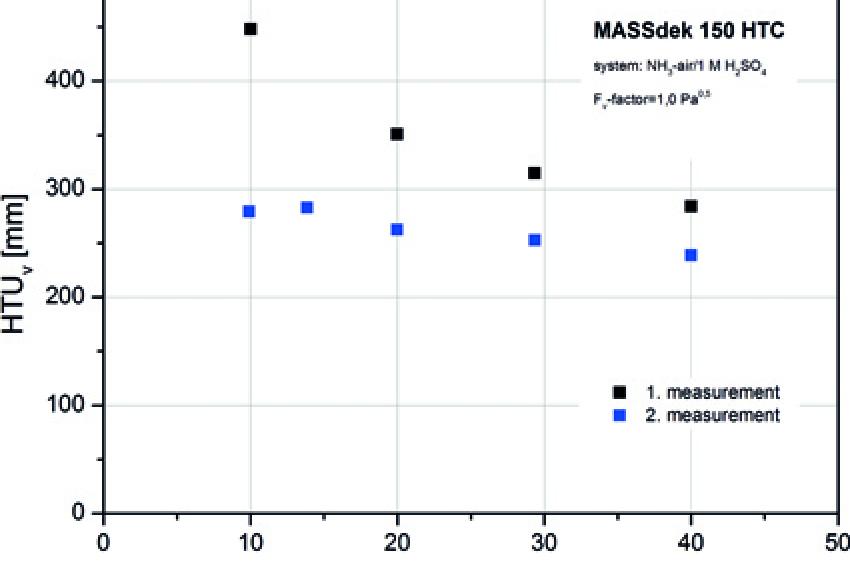
The same tendency can be observed in mass transfer tests using the same type of packing from Envimac in the NH3 air system (cf. Fig. (3)).
The packing in the third measurement is in a used condition, whereas no sufficient surface energy had developed during the first measurement. Again there are distinct differences in the HTU value. The change in the HTU values was achieved exclusively by the continuation of the measurements in the same test column.
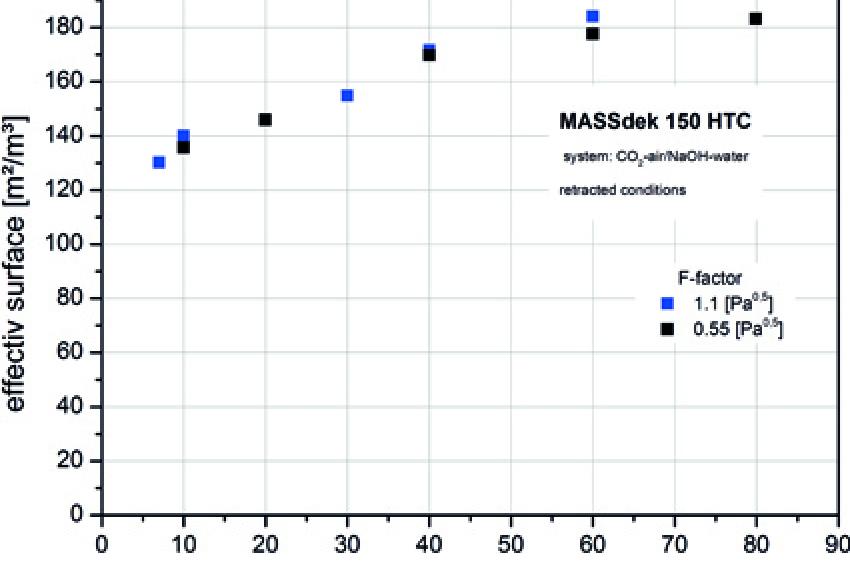
For this reason, it is very important to measure the performance of a structured plastic packing and the connected effective surface only a used condition with improved surface energy, not in a new condition. As shown, this condition develops in processes with aqueous systems in the course of operation. For the Massdek 150 HTC packing (geometric surface of 150 m²/m³), the effective surface of the filler was determined for used packing on the CO2 air/NaOH water system (Duss, Meierhofer, & Nutter, 2001).
The dependence of the effective surface on the irrigation density is seen in Fig. 4. Given a used film type packing, a high effective surface is obtained, which in case of higher irrigation densities (at an F factor of 1.1 Pa0,5 as from an irrigation density of around 20 m³/m²h) may even exceed the geometric surface. With an increasing irrigation density the foil develops a higher waviness (Al-Sibai, 2004), which produces this additional surface.
Conclusion
The typical system effective surfaces develop only after a sufficient run-up time. Measurements in test columns as well as computational models have to be considered in this respect.
Contact
GEA 2H Water Technologies
Kalscheurener Strasse 92
50354 Hürth
Germany
+49 2233 6999 0
+49 2233 6999 520