The Pharmaceutical Supply Chain
Too much emphasis on price can lead to cutting of corners on quality
Mean and Lean - The pharmaceutical supply chain has gone through a dramatic transformation over the past few decades, with an ever-increasing number of players involved in developing, manufacturing, marketing, and distributing drugs. Today, companies in the pharmaceutical industry are frequently turning to Contract Research Organizations (CROs), Contract Manufacturing Organizations (CMOs), and Contract Research and Manufacturing Services Organizations (CRAMs) to fill knowledge and technology gaps and realize cost-savings.
It is no secret that the R&D-focused pharmaceutical industry is struggling with discovering new products and getting them through clinical trials. In order to bridge the gap, pharmaceutical companies are increasingly using complex R&D cooperation models where they work simultaneously with several partners, including academia.
When it comes to manufacturing, pharmaceutical companies must make a decision whether to invest significant amounts in developing the new capabilities with the right capacity available the right time, or to work with partners who already have the required capabilities and capacity available. Due to financial pressures and the need to prove favorable return on individual project investments compared to other projects within the company that also require investment, making a case for investment in new capabilities may be difficult. Additionally, as the R&D side of the industry is putting more focus on patient-specific medicines, the amounts of a particular product needed to meet market demands will likely decrease which may further push the scales in CMOs favor. An increasingly complex regulatory environment, associated with rising costs of maintaining compliance, is frequently cited as another reason for outsourcing.
It has been common for pharmaceutical manufacturers to outsource the manufacturing of older and less expensive products, thus making room in their own facilities for newer products and allowing for tighter control of proprietary technologies and know-how. Even in the absence of suitable products to fill the pipeline, outsourcing continues due to the difference in the cost of manufacturing the product as compared to the cost of buying it from a third party, frequently located in India or China.
Abbott was the powerhouse when it came to manufacturing erythromycin. For years, they continued making the fermentation-based product in their North Chicago plant despite having higher costs than their Chinese counterparts who were making material of lesser quality. Today, however, there are plenty of sources of acceptable-quality erythromycin available in China allowing Abbott to discontinue the manufacturing of this product. Last year, Abbott announced that it will shut down the North Chicago manufacturing facility.
Outsourcing decisions cannot be made lightly. Once you hand over manufacturing of your product to a third party, you relinquish a significant amount of control, even if you have excellent contracts in place. Most likely, you will not be the third party's only customer, meaning that they will have to juggle the needs of all their customers who often come from different cultures with different regulatory requirements which at times may be contradictory. And the third party will also have their own financial objectives and margin requirements which can lead to sacrifices when it comes to regulatory compliance.
Among generic drug manufacturers there seems to be continuous debate over the pros and cons of making active ingredients in-house versus working with third parties. An increasing number of companies do both. Having API manufacturing in-house gives you more control over pricing and intellectual property. But few, if any, generic drug manufacturers can make active ingredients for all the products in their portfolio, so they need to work with third parties regardless. Economies of scale may dictate manufacturing capacities that are too large for internal consumption, while finding customers may prove difficult; other competitors may choose to buy from independent API manufacturers rather than their competitors.
Of the 464 API manufacturers rated by Thomson Reuters as stablished or Less Established, meaning that the companies have experience with supplying active ingredients to regulated markets, approximately a third are pure API manufacturers with others associated with groups that are also involved with finished dose products. (Figure 1)
So, who should one work with?
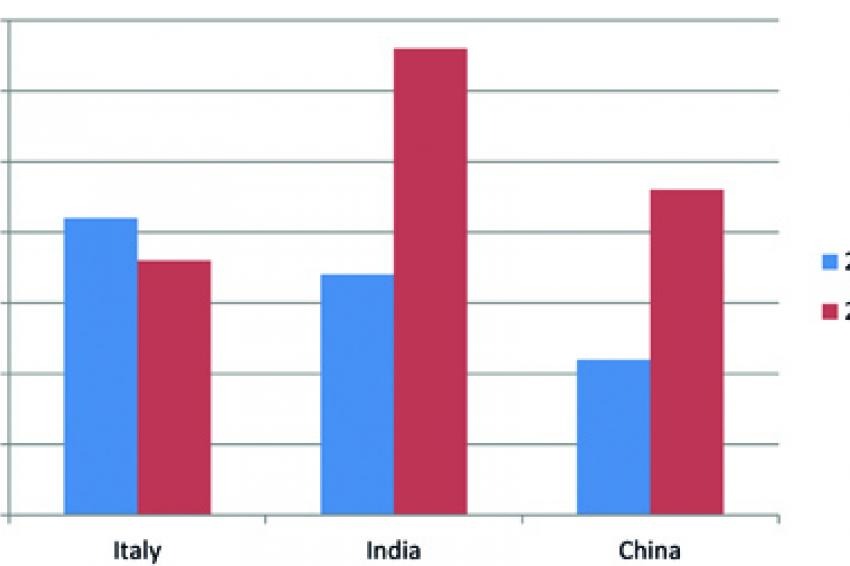
Both generics and Big Pharma are continuing to focus their attention on emerging markets to find low cost sources of raw materials, active ingredients and finished dose manufacturing. Among emerging markets, India is a popular destination for finished dose, while both China and India continue to enjoy a significant presence as outsourcing destination for API. The number of suitable sources of low cost active ingredients in India and China has increased considerably during the past decade. As seen in Figure 2, the number of API manufacturers rated by Thomson Reuters as Established or Less Established doubled in India and China since 2005. Meanwhile, the number of Italian-own groups has decreased, mostly as a result of acquisitions by foreign companies.
Meanwhile, the costs of manufacturing in India and China have increased as well: salaries are growing at double-digit rates, there is increased regulatory compliance, and environmental controls continue to be strengthened. If you add to the mix recent quality issues with both API and finished dose manufacturers and continued issues with IP protection, it is easy to see why some Western companies may be looking again toward their Western partners. A strong pipeline of oncology drugs that require complex and technically challenging HPAPI (High Potency Active Pharmaceutical Ingredient) manufacturing has also benefitted many of the more capable and experienced Western firms. However, contract manufacturers in North America have not been immune to regulatory issues either. For example, many pharmaceutical companies, generics, and API manufacturers have been hurt by Ben Venue's on-going troubles.
It remains to be seen what the impact of GDUFA (the Generic Drug User Fee Act) will have on the outsourcing decisions. The various fees, which are supposed to come into effect in Q4 of 2012, will apply to both finished dose and API manufacturers. Some of the fees will be linked to manufacturing facilities and will need to be paid every year, while others will be linked to individual filings, such as ANDAs and DMFs. It is likely that CMOs will pass at least some of the fees on to generic companies, so some generic companies may find it more attractive to work with manufacturing partners located in the US; facility fees for sites based in the US will be slightly lower than for over-seas sites.
As a result of GDUFA, contract manufacturers in emerging markets will no doubt face greater scrutiny and more frequent inspections than they have in the past. If regulatory compliance goes up as a result, some Western companies may be more likely to use them. On the other hand, especially in the short term, the risk of something going wrong during those additional inspections may increase, possibly scaring away some customers. It is also clear that compliance does not come cheaply, which may further diminish the cost differential between Eastern and Western manufacturers.
Outsourcing is here to stay. In order to gain the maximum benefit from outsourcing, companies will need to very carefully evaluate what to do in-house and what to outsource. Another set of important decisions surrounds who to partner with. Both decisions need to be based on core competencies and not just the price tag. Too much emphasis on price can lead to cutting of corners on quality which can lead to disastrous results for all involved.
Outsourcing arrangements are likely to become increasingly complex involving many parties in many parts of the world, so it is absolutely crucial that companies continuously invest in building and managing the outsourcing relationships. Clear lines of communication are crucial, but so is a system of multiple checks at multiple points in the process.
Contact
Thomson Reuters
215 Commercial Str.
Portland, Maine 04101
+1 207 8719700
+1 207 8719800