Impact of New Regulations on Global Antibiotics Production
14.06.2012 -
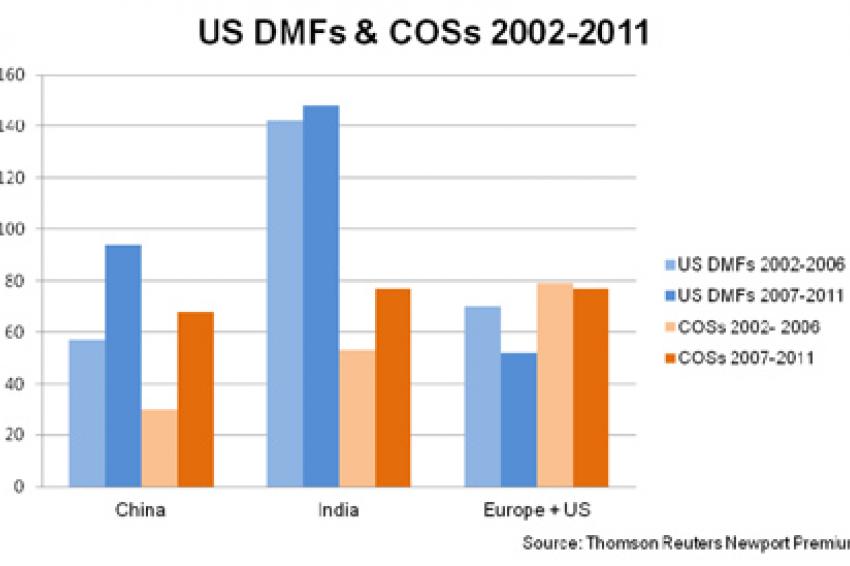
As companies continue to face ever-tightening budget constraints, many have turned to lower cost markets, such as China and India, as sources of active pharmaceutical ingredients (APIs). One therapeutic area that has seen much of its manufacturing transferred from the west to eastern facilities is antibiotics (See figure 1). In this respect, China and India have positioned themselves as leaders in the manufacture of materials for use in antibiotic products for many markets, including those in the US and Europe.
As the number of manufacturers in China and India continues to grow, their respective regulatory bodies struggle to retain adequate oversight of their activities. With potential impacts of companies' activities ever more scrutinized, governments have made moves to curb environmental pollution to help ensure the continued efficacy of available therapies and come into line with western standards. The effects of the actions they undertake could impact the global marketplace.
China, with its massive fermentation capacity, has become the top manufacturer of penicillin industrial salts in the world. Chinese suppliers are often able to offer these products at substantially lower costs than their competitors; this has led to decreased competition as many companies outside of China have reduced capacity or ceased production entirely.
As a number of antibiotic products utilize penicillin salts as a starting material, any substantial change to the manufacturing landscape in China has the potential to impact API and finished dose (FD) prices for companies sourcing from China. The impact of this lack of reserve manufacturing resources, coupled with increasingly stringent regulations, was evidenced a few years ago, when a number of Chinese antibiotics producers were told to close their doors.
The closures in China resulted in the price of penicillin G, a key intermediate for a number of antibiotics, including amoxicillin and a number of cephalosporins, tripling in a matter of months.
The Chinese government has reportedly imposed the strictest regulations to date, with sweeping changes being made not only to production standards, but also to antibiotic prescribing and consumption guidelines. The stricter Environmental Health & Safety (EHS) regulations implemented in China focus on several areas, including wastewater treatment. In traditional fermentation, like that of penicillin, vast amounts of wastewater are generated during production. Some of this wastewater is contaminated with antibiotic residue when it is released. The presence of these products in the environment has been linked with a growing global trend of antibiotic resistance.
This poses a threat to public health as organisms exposed to this residue garner additional defenses, allowing them to defeat a growing number of treatments. Under the manufacturing regulations, plants unable to meet the specifications for things like waste water contamination are facing complete closure or costly upgrades to existing facilities. In 2011, Harbin Pharmaceutical Group, one of China's largest penicillin producers, was fined by the Chinese government for its continued pollution of the water and air in the surrounding area, resulting in a cessation of API production. The company signed an agreement to relocate the plant in 3-5 years.
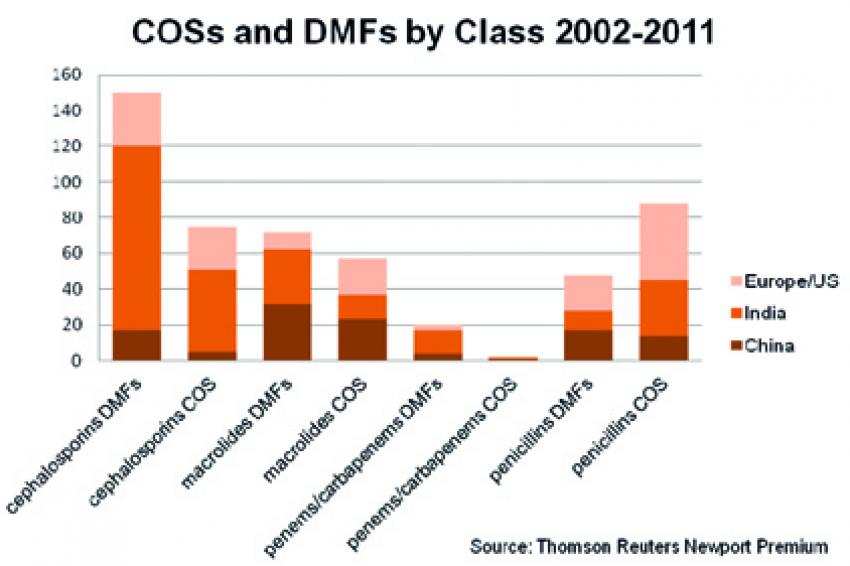
The Indian government has also announced plans to require compliance with environmental regulations, though there appear to have been fewer actions to date. Late in 2011, Orchid Chemicals' cephalosporin plant was cited for improper disposal of solid waste. This citation resulted in a facility shut-down lasting for several weeks. Recently, several plants were closed by the Pollution Control Board for violations, but details had yet to be released at the time of writing. With many products being sourced from China and India at various points along the supply chain (See figure 2), API and FD prices worldwide could see significant effects as the costs impact manufacturers.
While the additional cost of compliance for manufacturers in China and India could impact prices, it might also provide incentive for western manufacturers to increase their production of these products. Historically, the excess capacity and lower labor costs in China and India have led to lower cost products. As labor and energy costs rise in both countries, and more non-compliant manufacturers are being shut-down, western manufacturers could find greater opportunities for these products.
A number of manufacturers in Europe have recently made substantial investments in their European facilities for many therapeutic categories. Among them is GlaxoSmithKline who has said it will increase antibiotic production capacity at its Irvine, Scotland site to meet growing demand as part of a large scale investment plan in their UK facilities. As regulatory bodies look to implement stricter quality initiatives, this westward investment could become more prevalent.
Contact
Thomson Reuters
215 Commercial Str.
Portland, Maine 04101
+1 207 8719700
+1 207 8719800