Fighting Counterfeits
Enhanced Safety For Pharmaceutical Packaging
Protection - The seamless documentation of supply chains and the ruling out of counterfeit products as far as possible are measures that serve to protect producers as well as patients.
These functions are assigned to one- and two-dimensional codes with which pharmaceutical manufacturers in an increasing number of countries are legally required to mark their products. However, that is just one step.
Such an identification system must function across national borders and be adaptable to meet local requirements. A wide variety of peripheral equipment has to be integrated into an overall system, data processed, and existing machines suitably upgraded.
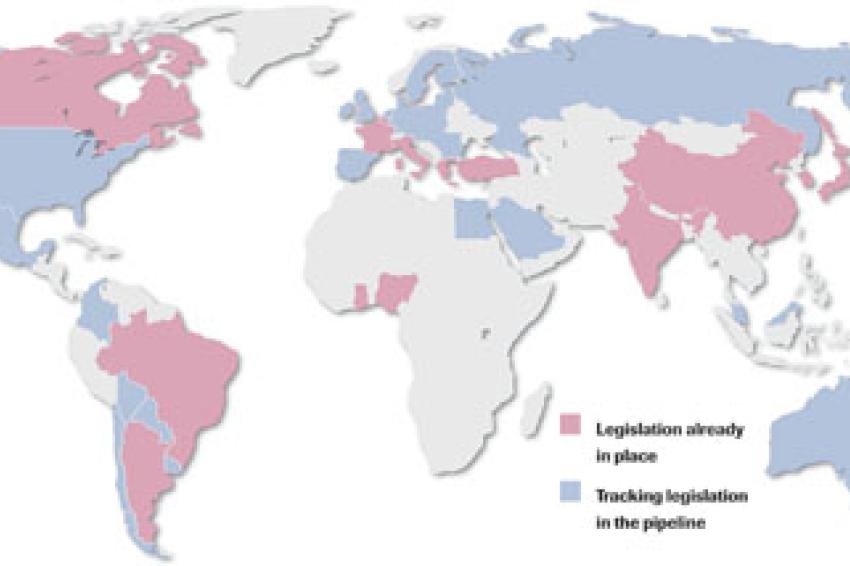
In several countries such as India, China, Brazil and Turkey it already applies; in others such as Russia, Spain and the U.S., it is in the pipeline: legislation governing the unique marking of the smallest unit of sale of pharmaceutical products with a code.
Problematic is the fact that the laws vary from country to country. While straightforward "French coding" by simply printing and verifying the code without data archiving suffices in France, in Turkey, for example, the verified codes have to be transmitted to a government database. The form of code also differs.
In some cases, a simple barcode (Code 128) depicting the data as a binary symbol is sufficient, whereas a data matrix code or 2D code encodes detailed information such as the product identification number, serial number, batch number and expiry date (YYMMDD) in a pattern of dots.
To ensure that coding is carried out properly, peripheral equipment such as camera systems and printers have to be purchased, installed in new and/or existing lines, integrated in a line database, and linked to the manufacturer's in-house IT infrastructure. This is a complex task that speaks in favor of an integrated solution with all components and services provided by a turnkey supplier.
Moreover, a solution based on a system that not only meets the requirement of marking the smallest unit of sale, but also offers benefits in terms of efficiency of the complete packaging process.
Competence from a Single Source
Track & Trace by Uhlmann is a joint development with VisioTec, Uhlmann's center of excellence for pharmaceutical inspection and printing systems. Track & Trace is implemented in three steps: Uhlmann VisioTec first provides the peripheral equipment such a laser or ink-jet printer and inspection systems for the verification of the codes and competently integrates these into the packaging lines. The second step involves the linking to a line database.
In the third step, the line data are transferred to a higher ranking system of the pharmaceutical manufacturer, the site server. Uhlmann has its own site software solution or cooperates closely with IT specialists that have established software systems for such applications. These systems also take over the processing of the data for the company network and the transfer of data to the authorities.
To ensure the ideal integrated solution from the packaging line to the internal ERP systems, Uhlmann maintains overall responsibility for the project and is the sole contact partner for the customer. This is the case no matter whether the system is integrated in an Uhlmann line or in packaging lines of other machine manufacturers.
Parent-Child Relationship
Track & Trace by Uhlmann works on the principle of parent-child relationship. Every element is marked with a code from the smallest to the largest packaging unit. A tracking database supplies the data to higher ranking systems and links the information for maximum pharmaceutical reliability.